Introduction
Evidence on patient preferences is increasingly being used to inform regulatory and reimbursement decisions. In acute leukemia, the number of treatment options is expanding but there is limited quantitative evidence on patients' treatment preferences, especially in the event of relapse.
The Acute Leukemia Advocates Network (ALAN) in collaboration with The Office of Health Economics are running a quantitative patient preference study using a discrete choice experiment (DCE) to 1/ Elicit adult acute leukemia patients' preferences for treatment outcomes and characteristics in the event of a future relapse 2/ Explore the tradeoffs that patients are willing to make between different hypothetical treatments 3/ Describe preference heterogeneity in the patient population and 4/ Provide useful insights that could demonstrate patient value for a range of upcoming health technology assessments of relapsed/refractory treatments and guide innovation and development throughout the medical product lifecycle.
Here we report on two stages of qualitative research: 1/ Online bulletin boards (OBBs) to identify potential attributes for the DCE and 2/ Cognitive ‘think aloud’ pilot interviews to test participant understanding of the draft DCE survey.
Methods
Two structured OBBs were conducted - one each for acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). Each day, participants were asked up to four open questions on the following topics: “your diagnosis and expectation of treatment”, “your first experience of treatment”, “treatment following a relapse”, and “your treatment priorities”. Participants could see and respond to each other's answers. Three researchers acted as moderators and engaged in discussions where appropriate.
Following the design of the DCE survey, a series of online one-on-one think-aloud interviews with AML and ALL participants was subsequently conducted to pilot the DCE survey. Interviewees were asked to verbally reflect on their responses, with occasional interviewer prompts. The pilots were conducted in two separate weeks, with a week in between to allow for changes to be made.
All participants were recruited via ALAN and its members.
Results
A total of 12 AML and nine ALL patients took part in the OBBs, of which 15 were female. Age (range: 26-71; average: 51.1) and years of living with the disease (range: <3 to > 10 years) were varied. Both the AML and ALL groups agreed that the effectiveness of the treatment in achieving long-term stable remission would be most important. Severe long-term side effects would also be an important concern but many participants said they could put up with potentially severe short-term side effects for a good chance of long-term survival. Additional concerns were the length of hospital stays, availability of psychological/mental/emotional support, catheter-related pain and infections, and mode of administration (MoA). Several participants argued that an individualized approach to treatment is preferred.
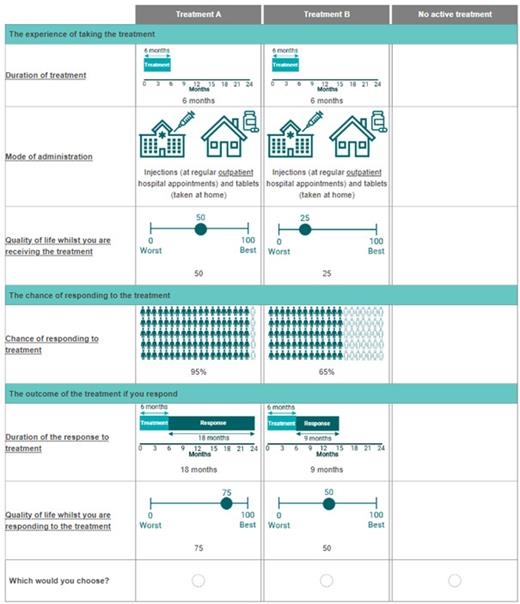
Thematic analysis of the OBB data led to the identification of five attributes for the DCE: chance of response (20-95%), duration of response (6-18 months), quality of life (QoL) during treatment (0-50%), QoL during response (25-75%), and MoA (tablets taken at home, injections requiring a hospital stay, injections as outpatient appointments). The DCE was designed using an efficient experimental design and coded as part of an online survey, and subsequently piloted (see Figure 1).
In week 1 of the pilots (n=5 interviews), ‘duration of response’, was often misinterpreted as ‘duration of treatment’. Interviewees also interpreted treatment and response as potentially overlapping, and therefore struggled to separate the two QoL attributes. Therefore, we made several changes including explicitly structuring the choice tasks to distinguish treatment and response phases. The changes improved understanding in week 2 (n=5 interviews) and raised some additional minor issues for consideration.
Conclusion
People with acute leukemia have a range of concerns about treatments in the context of a relapse. However, the primary issues identified in the qualitative research relate to the chance and duration of treatment success, QoL during and after treatment, and the MoA. The pilot interviews were valuable in improving the wording and overall quality of the final survey, which has since been fully launched.
Disclosures
Pemberton-Whiteley:Astellas: Consultancy, Other: Grant funding to the organization; AstraZeneca: Consultancy, Other: Grant funding to the organization; Autolus: Other: Grant funding to the organization; BMS: Consultancy, Other: Grant funding to the organization; Cancell Therapeutics: Consultancy, Other: Grant funding to the organization; Daiichi Sankyo: Other: Grant funding to the organization; Incyte: Other: Grant funding to the organization; Jazz: Other: Grant funding to the organization; Janssen: Consultancy, Other: Grant funding to the organization; Kite/Gilead: Other: Grant funding to the organization; Kura Oncolovy: Other: Grant funding to the organization; Pleco Therapeutics: Other: Grant funding to the organization; Kyowa Kirin: Other: Grant funding to the organization; Novartis: Consultancy, Other: Grant funding to the organization; Otsuka: Consultancy, Other: Grant funding to the organization; Pfizer: Other: Grant funding to the organization; Seagan: Consultancy; Servier: Consultancy, Other: Grant funding to the organization; Takeda: Other: Grant funding to the organization; Adaptive: Other: Grant funding to the organization; Glycostem: Other: Grant funding to the organization; Amgen: Other: Grant funding to the organization; AbbVie: Consultancy, Other: Grant funding to the organization; Leukaemia Care: Current Employment; INO Therapeutics: Other: Grant funding to the organization; Zambon: Consultancy. Nier:Astra Zenaca: Other: Grant funding to the organization; Autolus: Other: Grant funding to the organization; BMS: Other: Grant funding to the organization; Cancell Therapeutics: Consultancy, Other: Grant funding to the organization; Daiichi Sankyo: Other: Grant funding to the organization; Incyte: Other: Grant funding to the organization; Jazz Pharmaceuticals: Other: Grant funding to the organization; Janssen: Consultancy, Other: Grant funding to the organization; Kite/Gilead: Other: Grant funding to the organization; Kura Oncology: Other: Grant funding to the organization; Novartis: Consultancy, Other: Grant funding to the organization; Otsuka: Consultancy, Other: Grant funding to the organization; Pleco Therapeutics: Other: Grant funding to the organization; Pfizer: Other: Grant funding to the organization; Roche: Other: Grant funding to the organization; Servier: Consultancy, Other: Grant funding to the organization; Takeda: Other: Grant funding to the organization; Astellas: Consultancy, Other: Grant funding to the organization; Amgen: Other: Grant funding to the organization; Abbvie: Consultancy, Other: Grant funding to the organization; Kyowa Kirin: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal